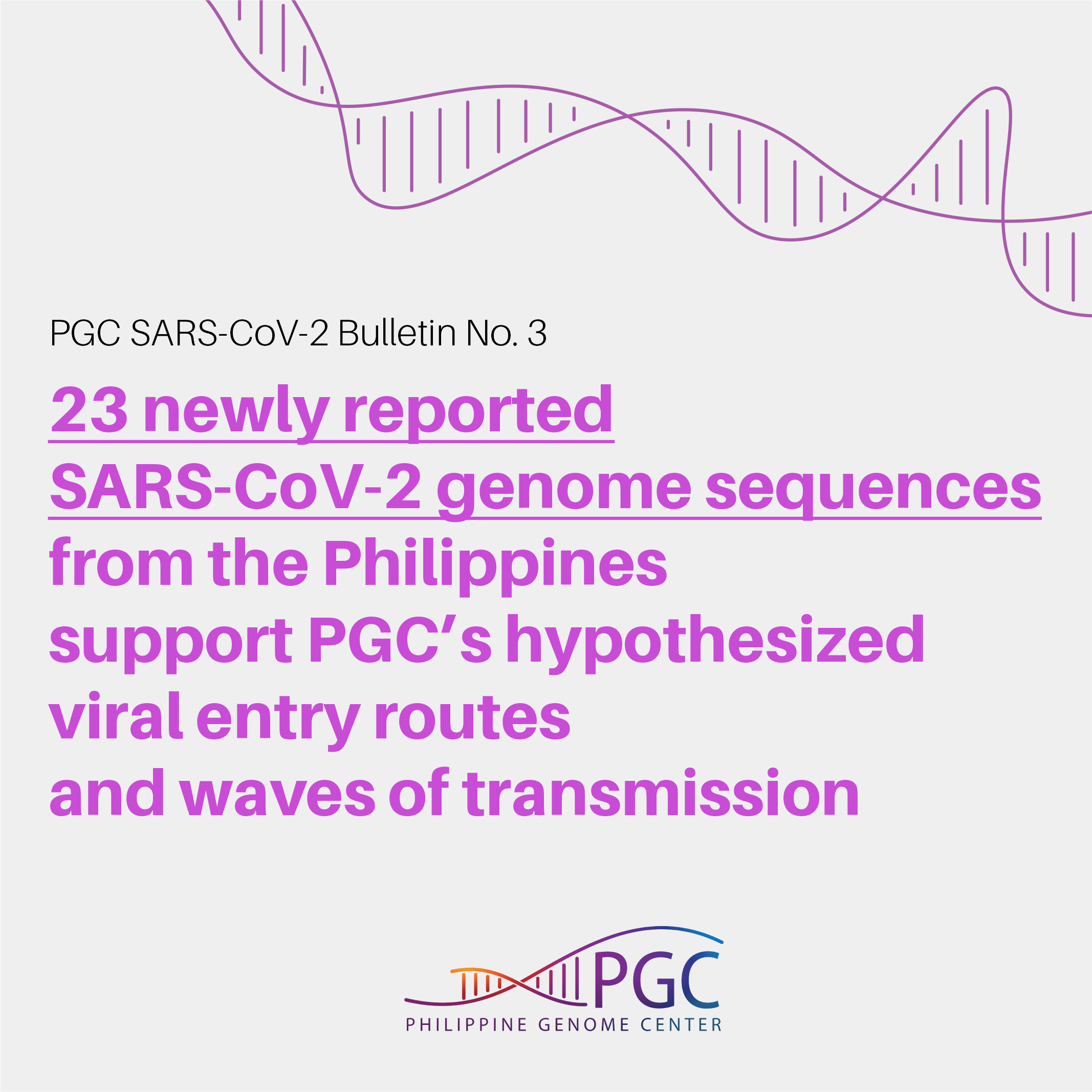
A group of researchers from the U.S. Army Medical Directorate–Armed Forces Research Institute of Medical Sciences, the University of the Philippines Manila, and the V. Luna Medical Center recently reported 23 new SARS-CoV-2 genome sequences from the Philippines, all of which were from cases of local transmission (Velasco et al., 2020). Among these samples, one was collected in early April while the rest were obtained in the months of June and July.

Updated rates for COVID-19 testing following the DOH & DTI JAO No. 2020-0001 starting Nov. 27, 2020
PGC’s Clinical Genomics Laboratory / COVID-19 lab releases updated rates for its swab testing services to reflect the base price of P3800.00 following the DOH & DTI Joint Administrative Order No. 2020-0001. The swabbing schedule runs from Mondays to Fridays, with the booth located at the Institute of Mathematics, National Science Complex, University of the Philippines, Diliman, Quezon City.
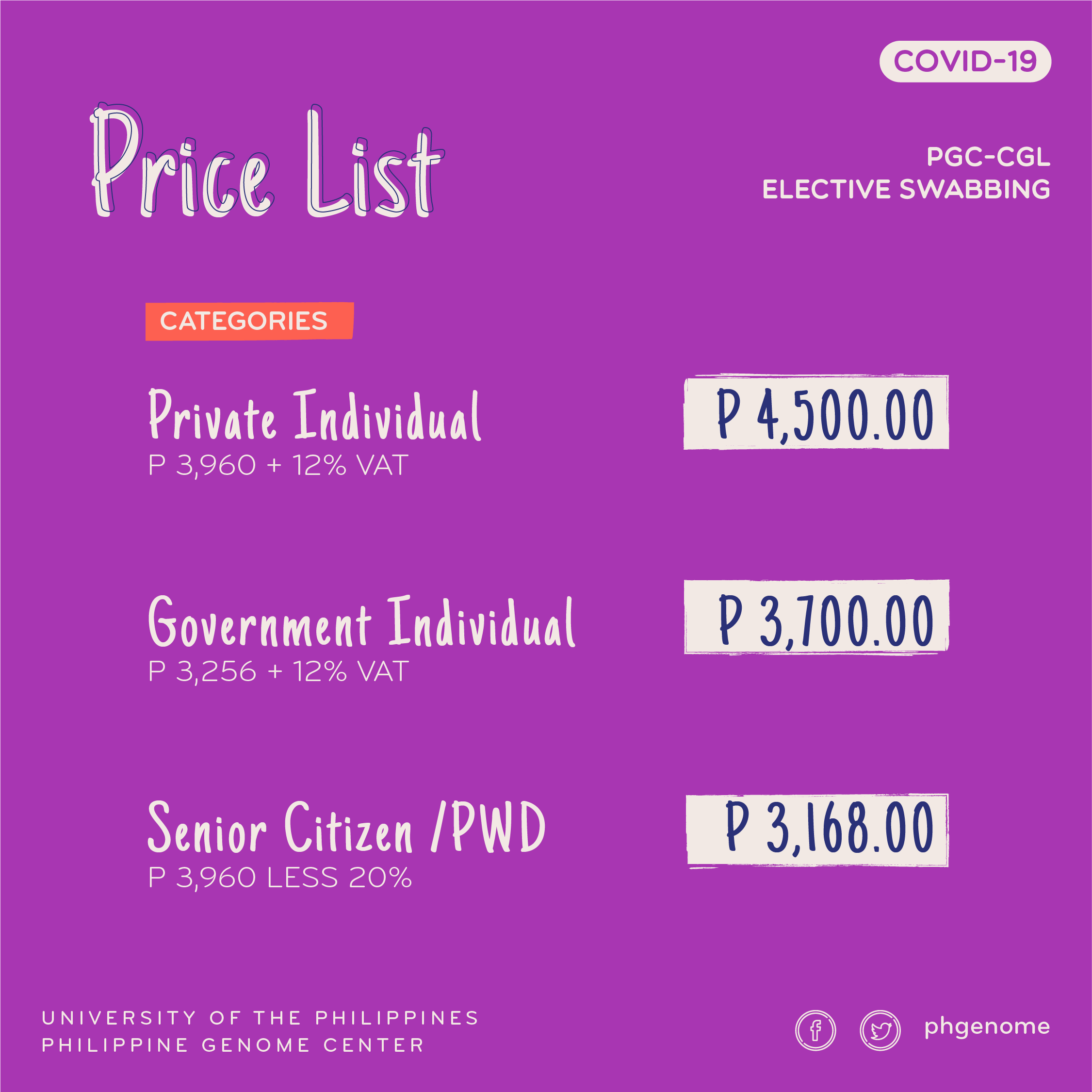
Updated rates for PGC-CGL’s COVID-19 expanded screening and drive-thru swabbing starting November 16, 2020
PGC’s Clinical Genomics Laboratory / COVID-19 lab releases updated rates for its swab testing services. The swabbing schedule runs from Mondays to Fridays, with the booth located at the Institute of Mathematics, National Science Complex, University of the Philippines, Diliman, Quezon City.

COVID-19 expanded screening and drive-thru swabbing at 2,280.00 Php (VAT exclusive) from October to November 2020
PGC’s Clinical Genomics Laboratory (CGL) is implementing a special rate of 2,280.00 Php (VAT exclusive) from October 14, 2020 until November 13, 2020 for all its rRT-PCR COVID-19 testing services. Similar process will apply on how to book swab appointments to prevent overcrowding and ensure that proper biosafety and security protocols are followed.

Clinical Genomics Laboratory as a COVID-19 lab and mass testing
PGC’s CGL Lab Manager and Clinical Health Officer Dr. Marc Edsel C. Ayes explains the function of the Clinical Genomics Laboratory and its role in the COVID-19 pandemic in the Philippines. Dr. Ayes also provides a brief explanation about Mass Testing.
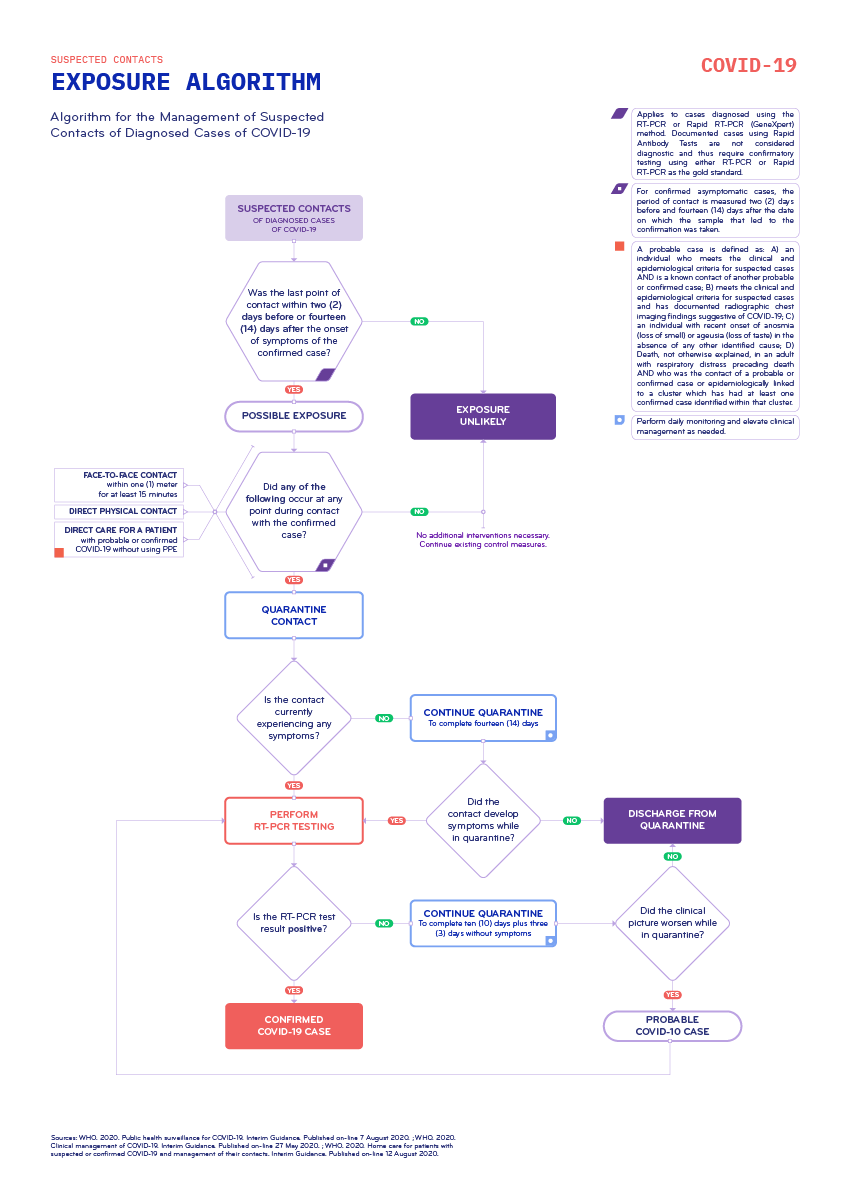
Contact Exposure Algorithm
The Philippine Genome Center’s COVID-19 Lab (Clinical Genomics Laboratory) developed a Contact Exposure Algorithm designed following the latest community guidelines of the WHO and CDC. The chart is intended to help inform the UP System Community with regard to conducting one self in the event of potential exposure. This algorithm may serve as reference for other communities, institutions, and/or offices.
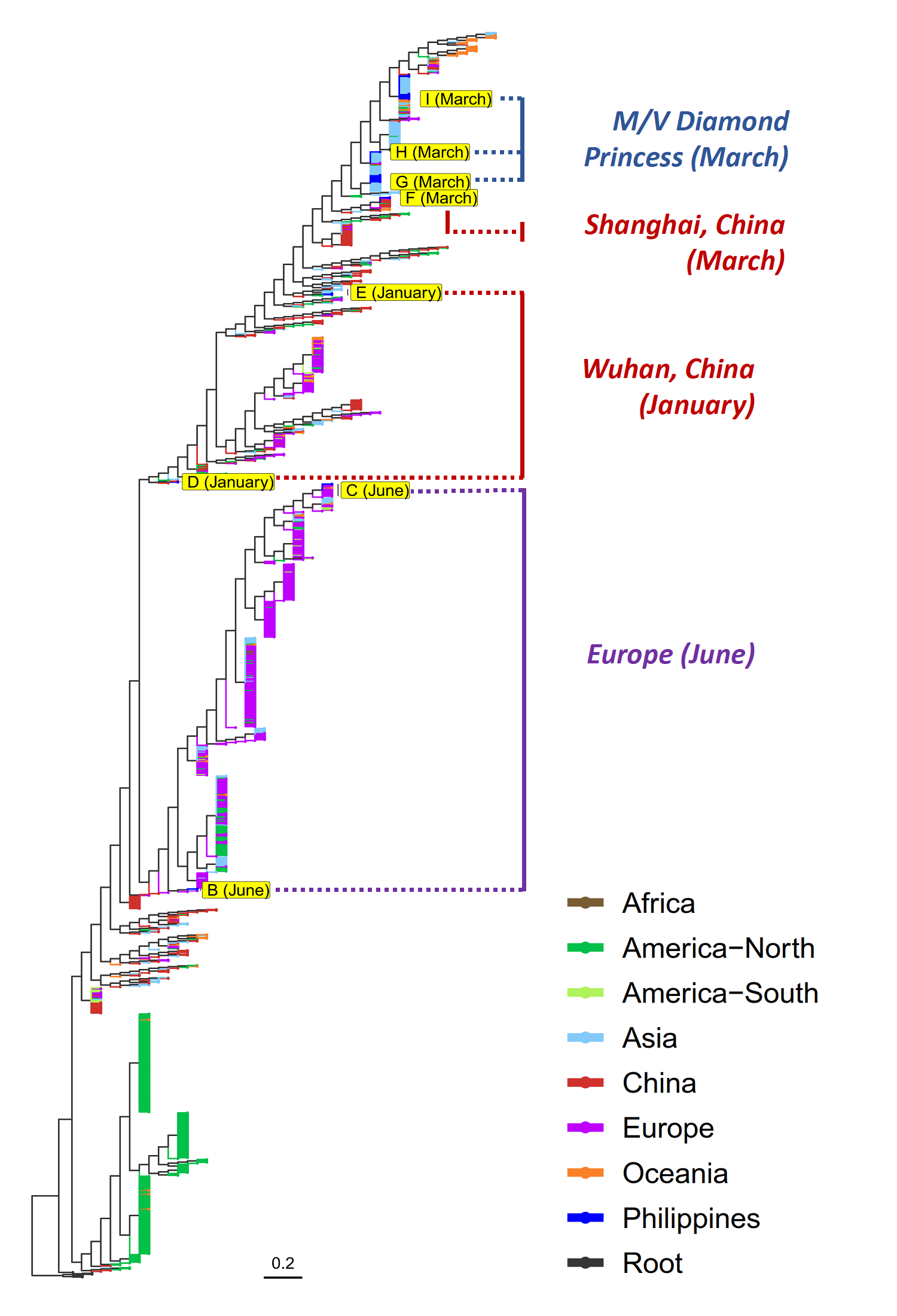
PGC SARS-CoV-2 Bulletin No.2: Three Possible Routes of SARS-CoV-2 infection in the Philippines
Majority of the Philippine submissions (18 of 23) were collected in the month of March, wherein except for one sample which clustered with isolates from Shanghai, China, all others were observed to group into clades linked to the outbreak in the cruise ship, M/V Diamond Princess, moored in Yokohama, Japan in early February 2020. Later that month, passengers and crew members of this cruise ship representing various nationalities including Filipinos, Indians, and Australians were repatriated to their home countries.
PGC SARS-CoV-2 Bulletin No.1: Philippine Genome Center Reports Detection of the D614G Variant of SARS-CoV-2 Virus in the Philippines
COVID-19 or the Coronavirus Disease 2019 is caused by SARS-CoV-2 virus, the genome of which is a single-stranded positive sense RNA that is about 30,000 bases long. It contains 11 genes and several regions have been known to be immunogenic, including different parts of the Spike (S) protein, the Nucleocapsid (N) protein, as well as the Membrane (M) and Envelope (E) proteins, which have therefore been targeted for vaccine development.

Artificial Intelligence-Assisted Loop Mediated Isothermal Amplification (ai-LAMP) for Rapid and Reliable Detection of SARS-CoV-2
Until vaccines and effective therapeutics become available, the practical way to transit safely out of the current lockdown may include the implementation of an effective testing, tracing and tracking system. However, this requires a reliable and clinically validated diagnostic platform for the sensitive and specific identification of SARS-CoV-2.
PGC’s COVID-19 Laboratory is a non-hospital based facility (NHB)
PGC’s Clinical Genomics Laboratory certified to conduct testing for COVID-19 (SARS-CoV-2) by Real-Time PCR is a non-hospital based facility (NHB)